6. Atom Constitution
(Page first posted September 22 2012)
Surprisingly, when combined, 2 of science unrelated facts do lead toward a physical fabric of the atom; a fabric consistent with gravimotion′s motion interpretation of Nature.
In the end, the atom has a physical constitution while remaining under quantic jurisdiction.
Wavelength to frequency disparity (§6.1)
The frequency of an electromagnetic wave is the number of periods per unit time, on the other hand the number of wavelengths per unit length, in physics science, doesn′t coincide to the frequency. Considering the red light of frequency 450THz, its wavelength is 660 nm; yet the number of wavelengths per meter: 1/660 10-9 = 1.5 MHz only; that is a frequency in space roughly 300 million times smaller than actual frequency in time.
A point of physics that does not make sense and kept hidden!
Unrelated observation (§6.2)
Because the speed of light C is a constant, one may choose a specific unit system in which that speed is 1, and Einstein famous equation becomes: E = m showing that mass is pure energy.
Bring mouse pointer here: conventions reminder.
Because empirical, physic′s mathematics cannot be denied. On the other hand the interpretation in words of these very undeniable mathematics may vary widely.
This internet site is about 2 different interpretations of physics′ mathematics:
Everybody′s interpretation, which is printed in blue color.
Gravimotion′s interpretation printed in black.
This internet site is about 2 different interpretations of physics′ mathematics:
Everybody′s interpretation, which is printed in blue color.
Gravimotion′s interpretation printed in black.
Wavelength frequency reconciliation; Introduction of the SI-ID system of units (§6.3)
I was looking for a model of the hydrogen atom in terms of physical trajectories when physics′ above 2 particular subject items came to my mind.Because the speed of light C = λ / τ , in which λ (lambda) and τ (tau) are respectively the wavelength and time period of the wave, when C is chosen equal to 1 (one), then:
C = 1 = λ / τ
as a consequence λ = τ and because τ = 1/frequency then λ = 1/frequency.In a unit system in which C is chosen to be 1 the wavelength λ is also the inverse of the frequency!
In science the frequency of an electromagnetic wave is exclusively the number of periods per unit time; in gravimotion the frequency is also the number of wavelengths per unit length and that is definitely not the case in science (§6.1 above).
A unit system will satisfy the condition C = 1 provided the unit of distance has been divided by the number of unit distance travelled by light in one unit time. Going ahead I am proposing a unit system called SI-ID, in which ID stands for Integrated Distance. The unit of distance in the SI-ID system is one meter divided by 299 792 458 that latter number being the number of meters light is travelling in 1 second; and let us call the SI-ID unit of distance im for integrated meter.
Because the red light 660nm wavelength mentioned above is λ = C/frequency the evidence is that its conversion in the SI-ID system is: λ = 1/frequency.
Note that in science, the meter has also been redefined in terms of light speed. Yet the new definition provides a meter having the exact same length as the previous definition.
The goal in science was not to change the length of the meter, as I do here; the goal was instead to be able to build a more accurate prototype of the meter.
Note also that while I make C=1, C remains nevertheless a speed; that is absolutely different than the suggestion made in science, in which while making C = 1 in Einstein′s famous equation (§6.2 above), "C" disappears, and in the SI system "m" miraculously becomes Joules instead of kilograms; making of mass another of those dubious dualities that exist in science.
A quick history of the quantized atom (§6.4)
- Johann Jakob Balmer first came up with an as brilliant as empirical formula, essentially introducing mathematically rather than experimentally the quantum aspect of an atom as soon as 1885.
- Zeeman disclosed its "anomalous effect" also before any one knew about the real constitution of the atom. It concerns single spectral line splitting into 2 separate lines. An effect that troubled scientists up to Pauli in 1924
- Finally some light was thrown onto the atom when JJ Thompson in 1897 discovered the electron, and measured its charge/mass ratio, which is a huge number.
- Then on a totally different subject, using Boltzman entropy concept and statistics, Planck explained in 1900 the radiation of black bodies; yet to do so he had also to introduce (invent) the concept of quanta.
- At that time the discovery of the photoelectric effect, could not be explained with Maxwell equations (dating from 1850) and Einstein, using Planck′s quanta, suggested that light is made of "packets of energy" now called photons. These photons physically hit electrons within a photoelectric material and knock them off. That was 1905.
- J William Nicholson 1911, 1912 showed that the angular momentum of a circling electron could only be an integer multiple of Planck′s constant.
- In 1913, Bohr′s introduced is famous atom and energy levels
- Yet new measurements were not explained by Bohr′s mathematics. And Sommerfeld suggested the electrons were riding ellipses instead of circles in special circumstances.
- Then came the Compton Effect, which allegedly proves beyond any doubt that light is made of photons.
- Once the wave/particle duality of light had been established, De Brogglie came up with the particle/wave duality of matter particles.
Essentially suggesting that electrons within atoms were some sort of voluminous vibrating clouds rather than discrete particles. - And Pauli in 1924 came up with his "exclusion principle", which solved Zeeman dilemma above.
- ...
- Gravimotion′s atom described below appears in 2012 with the first posting of this page.
In gravimotion the electrons ride physical orbits, rather than so called "quantic" mathematically defined "orbitals", which, exquisite mathematics set aside, transcend physical reality.
Gravimotion′s version of the atom is described in terms of Balmer and Rydberg formulas, to which is added Sommerfeld idea of ellipses.
The introduction of the SI-ID unit system (§6.3 above) merges quantum metaphysics and physical physics.
Oddities of Bohr′s atom (§ 6.5)
Should you refresh your memory, as I recently did, about Bohr′s atom, some odd things will make your mind wonder.
In the following gravimotion interpretation of the atom, the orbitals that coincide to higher and higher energies have smaller and smaller trajectories instead and all of them fit tightly within the outer atom orbit; the latest (physics lower energy state or ground state) being now the largest orbit on the graphical representation.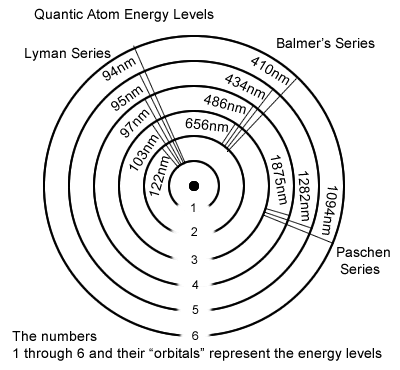
Figure 6.5
- The higher the energy level the larger the representation of its coincidental orbital; orbital #6 is larger than orbital #5, expressing that state #6 is more energetic than #5; and state #5 is more energetic than #4 etc. The so called ground state in the hydrogen atom being represented as having the smallest orbital (#1 on the graph).
This representation allows the atom to fall from a high energy state back to a lower energy state, when it is excited.
Even though that makes sense energy wise that is in conflict with item 2. next. - In accordance with Heisenberg Uncertainty Principle, the closer an electron gets to the proton, the better defined is its position, and the higher are its energy level and agitation, which keeps it away from the proton; and that explains why, in physics, the electron doesn′t fall on the proton, to which it is strongly attracted.
- As such Bohr′s atom, in which the closer to the nucleus is the electron the lower its energy level, contradicts the uncertainty principle, in which instead the closer to the nucleus is the electron the more energetic it is; amazingly these electron′s 2 incompatible behaviors are both mandated and honored in science!
Once I gave a written pamphlet about gravimotion to a physicist; he told me that he would look at something that is not physics′ science only if there were no contradiction in it. And he never got back to me. Strangely enough, since then I noticed there are a lot of annoying contradictions within physics′ science!
Interestingly enough that is not in conflict with physics own energy levels when these levels are considered to be equivalent to frequencies; as higher frequencies coincide to lower wavelengths. And to come up with a representation in which the discrepancy between items 1. and 2. no longer exist, one has simply to make coincide electrons′ trajectories and wavelengths!
In effect gravimotion′s interpretation replaces the metaphysical quantum states (figure 6.5) by simple physical paths, which lengthes nevertheless obey quantum rules!
Another fact that even some physicists are uneasy about is that when an electron jumps down from a higher level of energy to a lower level of energy in an atom, it does so instantaneously and disappears from reality as there are no allowable state in between.
Yet it is during that moment that doesn′t exist that a photon is emitted.
In gravimotion′s following interpretation, the atom emits light while in an existing that is permitted even though unstable state, and which is maintained by an external contribution, at the image of a glass of water, which when full is overflowing if the faucet is not shut off.
Finally there are the following 2 dilemmas.
- While electromagnetic waves seem to act as if they were made of quanta (photoelectric effect) that doesn′t mandate that light is made of quanta. Yellowstone Old Faithfull Geiser, emits in steps huge quanta of water. These amount quanta (the eruptions) are very likely the result of a progressive pressure build up lasting 20 min, rather than the result of an instantaneous (quantum) phenomenon.
- And even though that is in conflict with De Broglie matter duality, the behavior of the electron as a wave does not necessarily mandate that matter be made of waves. In the spirit of Bohr correspondence principle, which in essence states that rules that apply at the atomic scale must not conflict with those at the macroscopic scale, particles electrons are very likely not made of electromagnetic waves, just as boats are not made of the water waves they generate.
A new, yet inspired by Sommerfeld, concept of atom (§ 6.6)
The model of atom proposed below follows Sommerfeld′s proposed model in the sense that ellipses are involved, even though such model is now out of consideration in physics. While Bohr-Sommerfeld atom was unable to explain the Zeeman and Stark effects, gravimotion′s addition does.Just as done in physics theory, this page description is restricted to the hydrogen atom.
- Electron within the atom
In this interpretation the electron is a physical particle; its electric charge has a physical volume; and that physical charge is circling the proton nucleus. The electron effect is a magnet. - Proton
The proton also a physical particle, instead of staying put is moving and wobbling at the image of a distant sun, which wobbling does indicate it has a satellite. The proton effect is also a magnet. Evidently the electron / proton pair moves in unison due to their own magnetic fields rather than gravitational fields.
While Sommerfeld′s atom involved the motion of the electron, as far as I know it did not involve the motion of the nucleus. That represents a major departure from Sommerfeld′s atom; it has drastic consequences on our interpretation of quantum mechanics entanglement phenomenon for instance. - Electron and proton electromagnetic fields
While the electron and proton charges are equal, the motion of the proton follows a much smaller circular pattern and takes much sharper turns, creating as strong a field as the field of the electron, even though the latter is on a remote orbit in comparison.
To start with we consider that the magnetic fields created by the proton and the electron are equal.
And because the 2 fields are created by opposite charges moving in unison (in the same directions), while equal in strength these fields are also in constant antagonism. - Electron and proton fields when the atom is not excited
When an atom doesn′t emit light, these 2 electromagnetic fields, not emitting light are purely magnetic fields. As such, when a hydrogen atom is not emitting light, both electron and proton ride perfect circles and are moving at constant angular speeds, as that is the characteristic of magnets as pointed out in§5.3.4.Magnets are characterized by constant currents (§5.3.4)A magnetic field is the effect of a constant current...
...
A constant current of protons means that the protons have a constant motion thrust, while their orientation may also either constantly change (when riding a circle), or not change at all (when riding in a straight wire).
In other words a constant current may occur in a coil or within an atom.
...
The electrons constant motion mandates the electrons trajectories are circles and not ellipses.
Should the electrons ride ellipses their orientations′ (formerly constant) variation (while riding a circle) would now vary, and the equivalent current would no longer be constant but varying.
While they do not emit light, the 2 magnetic fields on the other hand strongly interfere with each other (§5.4).
Moving in the same direction, the proton and the electron create magnetic fields orientations that are opposite to each other; the wobbling motion of the proton is equivalent to a magnet within an equivalent magnet (that of the electron) but turned upside-down. It is as if the two (faked) magnets of figure 5.4.4 were overlapping each other, being intricately interlaced into a strong and inescapable embrace.
In the end the motions of the electron and of the proton create 2 opposite yet complementary magnetic fields, which fuse them together in an un-escapable merry-go-round dance. - The atom resonance
Because both electron and proton move in unison (item II above), because the fields they produce do match (item III above), because these fields do interfere, and because their orientations are opposite and complementary (item IV above), these 2 fields along their particles proton and electron enter in resonance.
As a general rule, the resonance phenomenon involves a physical entity, such as a cavity, a guitar string, or an LC (inductance, capacitor) circuit, which no matter the entity has a natural vibration frequency.
In the case of the atom the 2 antagonistic yet interlaced fields constitute the harmonic oscillator; the motions of the 2 particles defining the vibrating frequency of the whole resonating system, and at same time the size of the atom. - Pauli′s exclusion principle
Such a resonance mandates the following:- That resonance frequency is uniquely defined by the electron and proton respective declivity and acclivity (§4.10), their respective motions and engendered fields and also the fabric of space (§3.1).
As such the resonance frequency is unique. - By the same token, because that resonance frequency formats the atom, the atom size is unique.
- That resonance frequency being unique also determines the only state of the atom, which in Bohr′s mathematics is all together allowable and stable.
In favor of gravimotion, note that in physics besides mentioning that that state is the "ground state", no physical explanation is given for it.
Neither the electron orbit (its altitude) nor the proton much smaller circular trajectory can be changed without external interaction. Should the electron want to change orbit on its own, moving either outward (on a larger orbit) or inward (closer to the proton), it would coincide to a change of frequency (energy in physics), which in both cases involves external interaction. - Finally that resonance being unique can share neither another electron nor another proton.
That resonance, which involves a specific and unique situation, substantiates physically Pauli′s mathematical exclusion principle.
- That resonance frequency is uniquely defined by the electron and proton respective declivity and acclivity (§4.10), their respective motions and engendered fields and also the fabric of space (§3.1).
- Unstable state
When an external contribution (motion in gravimotion, energy in physics) is provided, the atom is thrown out of equilibrium that is out of its resonating frequency (out of the ground state in physics) and finds itself in an unstable state.
Yet while in physics the atom then falls back instantaneously toward its stable state, and at same time is emitting a photon, in gravimotion the atom instead is maintained in that unstable state by the external contribution; the atom, which cannot absorb that contribution, emits an electromagnetic wave (spills over the energy it is provided), and as already mentioned does so at the image of a glass of water, which when full is overflowing if the faucet is not shut off.
In gravimotion the external contribution (described in details next) is lasting in motion (is not instantaneous), and its effect (emission of electromagnetic waves) is also lasting (rather than being instantaneous as is the photon emission in physics). -
Excited state that is unstable state (VII above) yet lasting over time
Next we focus on that external contribution and the effect it has on the atom.
Because it allows to precisely measure the spectral lines emitted, in physics experiments, the external contribution is either occurring through photons (light rays) hitting the atom, or particles electrons or particles protons hitting the atom.
In this essay though, we take advantage of physics measurements while considering a different situation; we consider the hydrogen atoms that are in our sun, which also emits light; and atoms in the sun are thrown out of equilibrium neither by particles photons nor by electrons nor by protons, but through the combined gravity of the entire sun; and the closer to the center the higher the effect of gravity; the higher the pressure on the hydrogen atoms (spherical to start with), the more ellipsoidal (flattened by gravitational pressure) their individual volumes; in the end the higher the pressure onto 2 juxtaposed hydrogen atoms, the higher the probability they fuse together into a helium atom.
Right here in our sun, hydrogen atoms are pressurized physically through gravity, and pairs of atoms end up fused into single helium atoms.
And by the way this evident interpretation that puts gravitation at work, is in conflict with physics very mathematics; in quantum physics, at so called quantum scales, the force of gravity is negligible; the strong force instead is in force!
In the process of fusing the hydrogen atoms, and before these get fused into helium, the radial-activity (the force of gravity) compresses and distorts these atoms.
Both electron and proton trajectories, which are perfect circles when the atom is in a dynamic equilibrium, are turned into ellipses by the radial-activity at same time throwing the atoms out of equilibrium.
The idea is not of mine. The ellipses were suggested by Sommerfeld; most importantly while riding ellipses electrons are accelerated and decelerated twice each circumvolution!
The distinction between the non-emission and emission of electromagnetic waves by an atom becomes crystal clear.- In the first case the electron rides a perfect circle, in the second it rides an ellipse.
- In the first case the electron′s motion is constant (as described in §5.3.4), in the second it is accelerating and decelerating twice along each full circumvolution.
- In the first case the electron produces a magnetic field, in the second it is also generating electromagnetic waves.
- In the first case the electron and proton are in a state of resonance (items V and VI above), in the second they are forced to both vibrate in symbiosis yet at 2 different complementary frequencies.
- The higher the compression due to the sun′s gravity, the shorter the ellipses perimeters as compared to the electron original trajectory that is as compared to the outer circle coinciding to an unexcited atom.
- The higher the pressure, the shorter the ellipses, the shorter the wavelengths emitted; in the end, because the wavelength is the inverse of the frequency (not the case in science physics §6.1 above), the higher the pressure, the higher the frequencies emitted.
By contrast the radius of the atom associated to so called "quantic orbitals" increases along the energy levels, and along the frequencies, in physics′ representations of Bohr′s atom; an anomaly noted in items §6.5 above.
Gravimotion′s excited atom is not bigger but smaller than the atom at rest, and that takes care of Bohr′s representation anomaly.
- Once through describing the constitution of the hydrogen atom in equilibrium or in a stable state (items I through VI above), and through describing the effect on that constitution by the outside contribution (item VIII above), which sets instead the atom out of equilibrium and its volume into an ellipsoid (flattened sphere) under pressure, let us focus on that non stable state of the atom, which in gravimotion is lasting a finite time under the influence of gravity.
The elliptic trajectory thought of by Sommerfeld, is precisely the missing piece that I was looking for to complete my interpretation in terms of motion-occurrences (§1.4), of the atom when emitting electromagnetic waves.
Furthermore it explains how light is emitted as a wave and on a constant basis rather than being emitted as instantaneous energy photons quanta. While these instantaneous quanta are a dilemma in physics and not needed in gravimotion, the so called quantized states of the atom remain untouched in gravimotion.
The simplicity of such a scheme is flabbergasting. All is needed in reality is an electron riding a suitable physical path, such that its motion is engendering electromagnetic waves!
While these physical trajectories belong to the physical world they nevertheless match the elusive quantic orbitals that belong to theoretical physics.
These ellipses are physically drawn at scale in §6.7 below.
Did you ever compare the emission of electromagnetic waves in the 2 cases that are the atom and a radio antenna? If you did, I did too and I am sure we agree. Obviously the electrons do not jump from one energy level to another while travelling in an antenna as they do in physics quantic atom. That is definitely another controversy of science. The above explanation, which takes in account the motion of an electron rather than its hypothetical jump from an energy level to another, reconciles the macro and quantum worlds. - Zeeman anomalous effect, which concerns the splitting of 1 line into 2 lines under the influence of a strong magnetic field, is explained as follows.
In section III above, we′ve seen that the electron and the proton respective motions create 2 antagonistic magnetic fields, yet in tune with each other and forming the resonance introduced in section V above. As such the interaction of an external strong magnetic field will be opposite on the electron associated magnetic field as compared to the interaction on the proton associated magnetic field.
That leads to the idea that the electron and the proton emit each a single frequency, both frequencies being in tune with each other when no magnetic field is present; and these 2 in tune frequencies separate, when a magnetic field appears. One frequency being emitted by the electron the other by the proton, as a magnetic field will have opposite effects on the electron and proton own associated magnetic fields.
As a reminder magnetic fields (or static electromagnetic fields) interact with each other (§ 5.4), but not with electric charges.
And electric fields interact with the motion of electric charges, such as protons and electrons, which indirectly modify the fields engendered by the particles.
Defining the physical ellipses within the atom(§ 6.7)
The final task consists in drawing ellipses, which perimeters are precisely equal in length to the wavelengths of the spectral lines observed in reality.The ellipses we are looking for, which do not exist in physics, are nevertheless given by Rydberg formula, which defines physics quantum energy levels and orbitals:
1/λ = RH (1/n′2 - 1/n2) (Rydberg formula).
In which n must be greater than n′, and n′ defines series of spectral lines called Lyman (n′=1), Balmer (n′=2), Paschen (n′=3) etc.
It must be mentioned that both Rydberg′s formula and constant have been "inferred" out of Balmer′s formula and constant, and that such inference emphasizes the geniuses of both Balmer and Rydberg:
λ = 364.50682 10-9 (m2 / m2 - n2) (Balmer formula).
Because the wavelength λ is our focus and not its inverse (the inverse being energy in physics yet equivalent to frequency in reality) the Rydberg formula is rewritten here:
λ = 1/RH (n′2 n2 / n2 - n′2)
In the SI system Rydberg constant for hydrogen is:
RH = 4 / 364.50682 10-9 m-1 (4 divided by Balmer′s constant in meter)
Evidently all of the following calculations concerning the ellipses perimeters and coinciding wavelengths are in the SI-ID system introduced in §6.3 above; and in the SI-ID system 1/RH becomes:
1/RH = 364.50682 10-9 / 299792458 x 4= 30.396597 10-17 im (in the SI-ID system).
Rydberg formula becomes:
λ = 30.396597 (n′2 n2 / n2 - n′2) 10-17 im, with n greater than n′ and n′ chosen to be 2 for Balmer′s series.
For convenience let us start with a single series of spectral lines; and there is a good reason (substantiated below) to choose the Balmer′s series.
The first table below converts the wavelengths of Balmer′s lines from meter to im, dividing the former by the number 299792458.
| Spectral line name | n (Rydberg formula) | Wavelength in meters (SI system) | Wavelength in im = wavelength (meter) / 299792458 (speed of light) |
|---|---|---|---|
| Hα | 3 | 656.21 10-9 | 218.888 10-17 |
| Hβ | 4 | 486.074 10-9 | 162.136 10-17 |
| Hγ | 5 | 434.01 10-9 | 144.77 10-17 |
| Hδ | 6 | 410.12 10-9 | 136.801 10-17 |
| ... | ... | ... | ... |
| huge | 364.56/4 10-9 | 30.396 10-17 |
Table 6.7.1
Balmer′s Hydrogen Spectral lines Wavelengths in im (integrated meters SI-ID system)
The perimeter of any ellipse can be calculated using the site cleavebooks.co.uk.
The terms used next are defined in figure 6.7.1
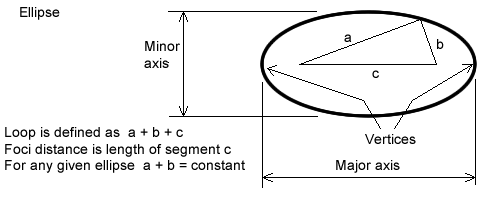
Figure 6.7.1
Ellipse′s parameters
Building the physical ellipses(§ 6.8)
Through several trials and errors I found it is convenient to choose a loop and give various values to the foci distance to get the right ellipse target perimeter (the wavelength).Through additional trials and errors, I inadvertently discovered that choosing Balmer′s constant for the building loop of the ellipses leads to very interesting results.
It must be mentioned now that in the final analysis, because both electron and proton travel at speeds lower than speed of light (which is 1) the trajectories found will have to be shortened by a relativistic factor. This being beyond the scope of this web-page will not be done here; but it has to be mentioned as the lengths of the trajectories calculated here are an absolute maximum.
Supposing as an example that the electrons ride at 90% the speed of light, then the trajectories calculated here would have to be shortened by 10%.
The next table and its animated illustration show the ellipses built using such a method.
The successive columns respectively provide the rank of the spectral line considered, its coinciding target ellipse perimeter length (actual wavelength measurement), the loop chosen to be equal to Balmer′s constant (expressed in im), the foci distance chosen through trial and error as to get the target perimeter, and the closest ellipse perimeter found.
The 2 last columns, major and minor axis, have been added as they show interesting results.
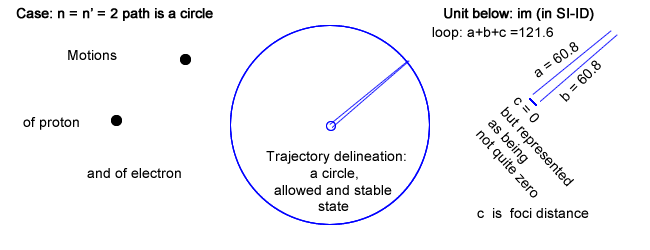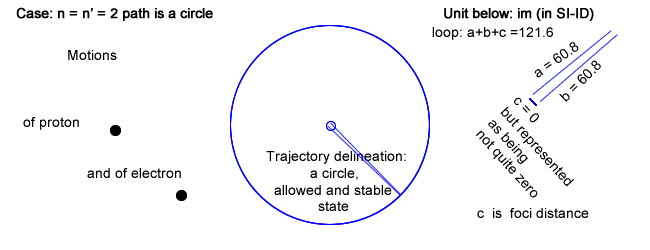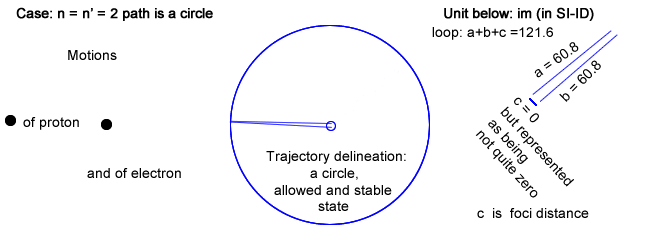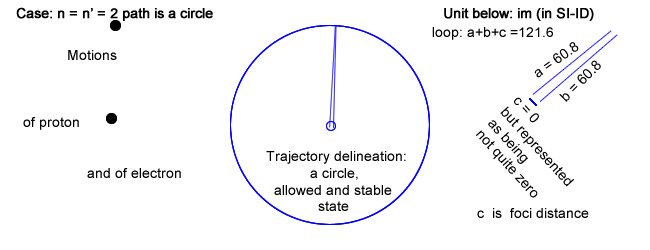
Please note that there is a row labeled n = n′ = 2 ; a case never mentioned in physics.
Bring your mouse pointer over the table and click "next ellipse" to see the illustrations coinciding to each spectral line.
|
Table 6.7.2
Physical trajectories and perimeters coinciding to the entire Balmer′s series
- First, and in addition of having chosen Balmer′s constant for loop, choosing n = n′, which is never done in physics, leads toward an infinite wavelength λ (no back and forth recurrence) and its inverse a zero frequency (coinciding to a constant current).
This situation, even though not considered in physics, can be physically implemented choosing c (the foci distance of the ellipse) equal to 0, in which case the ellipse is drawn as a circle.
That circle has a radius, which length is half the loop value, or half Balmer′s constant.
The electron rides a circle that is equivalent to a constant circling current that is creating a magnetic field (section IV above).
Even though the electron rides its trajectory repetitively, it doesn′t emit any electromagnetic waves as there is no variation in current.
The trajectory can nevertheless be calculated as: 2πr with r = Balmer′s constant / 2.
And that trajectory evidently coincides to the state, which in quantum physics is first allowable, second stable and altogether of lowest energy for that series (provided all series have a low energy state).
That physical trajectory is now represented as the largest of the other in the same series, and not the smallest "quantic energy orbital" as done in physics and as shown in §6.5 above (specifically level 2 for Balmer′s series).
And we always have in mind that a relativistic factor has to be applied to these results, which will shorten the physical trajectories lengths. - On the other hand (just as in quantum physics), for any given n′, choosing n huge in Rydberg formula leads to a shortest λ (maximal energy in physics and maximal frequency in reality).
In this new interpretation, in the case of Balmer′s series but also in all series, the c that is the foci distance of the ellipse becomes also the major axis.
Physically the length of this major axis is equal to half a wavelength and half of the Balmer′s formula constant.
The ellipsoidal trajectory is compressed to a maximum into a flattened line.
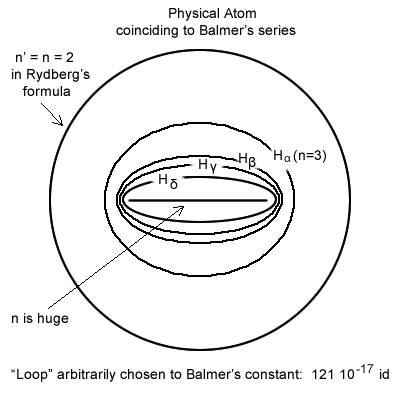
Figure 6.7.2
Then each series has its own enclosing spherical (circle) trajectory; trajectories that are not mentioned in physics science.
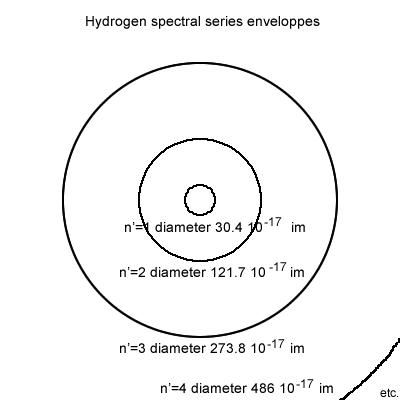
Figure 6.7.3
The trajectories perimeters (perfect circles) are calculated using the formula: 1/RH . n′2 = 30.396597 . n′2.
As an example for the Paschen series the circle diameter is 30.396597 10-17 x 9 ; "9" being n′2 with n = 3 for Paschen series.
In the case of the Lyman series (highest in energy, shortest wavelengths) should one make n′ = n the diameter of the circle becomes 30.396597 10-17 im, and the circumference is 95.493939 10-17 im; should the electron travel at speed of light that would coincide to 1 047 1012 circumvolutions per second.
Yet no wave is emitted, because a wave is only emitted through accelerations/decelerations; and when the electron rides a circle there is no acceleration/deceleration and the frequency emitted is null; the emission becomes a magnetic field. As a consequence that frequency of 1 047 1012circumvolutions per second actually emits a constant (magnetic field) rather than an electromagnetic wave.
The numbers n′ = 1, n′ = 2, etc. in figure 6.7.3 do coincide to physics quantum energy levels labeled 1, 2, 3 etc. in figure 6.5 above; yet in figure 6.5 neither the notation n′=1, n′=2, etc. nor the notation n=1, n=2 etc. (as usually done in treatises of physics) is used. The notation 1, 2, 3 etc. instead is used because these levels are ambiguous. Consider the wavelength 656 nm in Balmer′s series; its bottom level coincide to n′ = 2 in Rydberg formula, yet written as n=2 in Bohr′s atom traditional representation.
Furthermore its top level labeled n=3 (in traditional representations) coincides to n= 3 in the formula and not to n′=3; yet it is the level n′=3 coinciding to the lowest limit of the Paschen series that is represented on the graph (as n=3), for the top level of 656 nm.
An interesting comment is that these enveloppes are coinciding to a gravitational field; their "compression" occurs in a squared sequence of n; and that corroborates the specific situation of the atom chosen within the sun as described in this internet page §6.6.II and §6.6.VIII.
The shortcomings of gravimotion′s atom (§ 6.9)
Even though the electrons ride realistic paths instead of metaphysical orbitals, some mystery remains within gravimotion′s atom; it rests on the empirical formulas established in physics.More precisely, missing in gravimotion is the link between the resonating cavity forming the atom and the physical constitution of both proton and electron.
The question is: why is the hydrogen Hα wavelength precisely 656.3 nano-meter and not 656.4 for instance?
Another question remains unanswered, and that is: why is there several series, with different large stable trajectories (low energy level in physics) and different frequencies / flattened ellipses (high energy level) limits.
Yet these problems are part of physics′ science too. And because gravimotion′s interpretation only involves a change in the definition of the unit system, all of physics mathematics which is no doubt valid, applies to gravimotion′s interpretation; in the end the latter is as valid as the conventional interpretation made of physics mathematics.