Subatomic particles and atoms (§4.12)
A full description of the atom (Chapter 6) is subordinate to electromagnetic fields (Chapter 5). The simplest atom is the hydrogen atom made of a proton and an electron.
The simplest atom is the hydrogen atom made of a proton and an electron.An atom of hydrogen is made of an incompatible pair proton / electron (defined §4.1.6 items 5 and 6).
Figure 4.12.1 illustrates the "hole" of MOs (the electron), within the "heap" of MOs (the proton), the whole within neutral pure-activity (§4.1.6 item 8).
On one hand the proton electron strong reciprocal attraction (implemented physically in next chapter) maintains such incompatible pair bonded, on the other hand their incompatibility (§4.1.6 items 4,5 and 6) maintains their respective integrity. The two particles end up entangled in an unending dance.
Note that in physics the electron cannot fall onto the nucleus (positive proton) for another reason. In physics, should the electron get in contact with the proton it would then be in one place, but that would provide it with a maximum momentum (Heisenberg uncertainty principle), which momentum keeps it away!
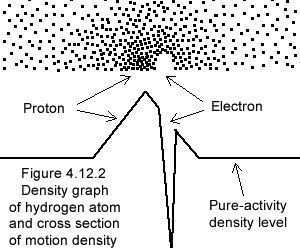
Furthermore (unlike physics), in gravimotion electron and proton enter in resonance; the frequency of which is also determined by the local pure-activity (the structure of space), with which a perfect match is in effect (§4.1.6). The whole dynamic structure proton/electron-pair pure-activity works in symbiosis and define the size of the atom.
Because the electron is a lack of MOs very little if any friction is involved and the process is practically eternal.
Looking at the density graph on the left one must imagine the electron frantically moving within the proton graded-activity, their intertwined motions forming the atom of hydrogen. Here also the individual MOs are wiggling around, and these graphs are drastic simplifications of reality as subjacent motions, besides the background pure-activity, are furthermore not represented.
More complex atoms containing more than one electron proton pair are resonating on other specific frequencies and have larger motion sizes (coincidental to physics energy states).
In physics, radiation energy originates from the atom when electrons are jumping from one energy level to another; and Planck's constant relates the radiated energy to its frequency by the formula Energy = Planck's constant x Radiation frequency, which also identifies energy to frequency.
An atom can be excited to an higher resonating frequency (physics higher energy level); the atom will then return to its original resonating state releasing some "frequency" in the form of electromagnetic wave, described in next chapter.
Because the jump of an electron within an atom, from one orbital (energy level in physics) to another, emits a frequency or an electromagnetic wave, gravimotionís interpretation, which is that the atom is occurring in resonating frequencies, makes utterly sense.